Defination:
process of mixing of orbitals of different shape and energy to form the orbital of same shape and energy .
Why hybridization ?
When we use to study a hyberdization ist question comes in mind why we are studing this topic .The answer is we are studing hyberdization because tetra valency of carbon can not be explained without this concept .This can be eplained if we see electronic configration of carbon .Atimic number of carbon is 6 its electronic configration would be 1S2.2S2.2px1.2py1.2pz0
According to this configration it can form 2 bonds but it forms four bond as in methane(CH4).
Types of hybridization;
It is of different type .
SP hybridization:-
Defination: It is a type of hybridization in which 1s and 1 p atomic orbital mix to form 2SP hyberid orbital.
Bond angle :its bond angle is 180
Shape :shape of sp hybridized molecule is linear.
Character percentage : SP hybridized molecule have 50% S character and 50% P character.
Example: Its example is ethyne and BeCl2.
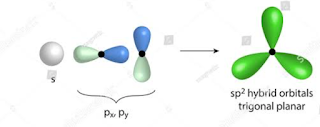
SP2 hybridization:-
Defination: It is a type of hybridization in which 1S and 2P atomic orbital mix to form 3SP2 hybrid orbital.
Example: Its example is ethene and Boron trifloride(BF3)
Bond angle : Bond angle in this type of hybridization is 120.
Shape: Its shape is Triangular planer.
SP3 Hybridization:-
Defination: It is type of hybridization in which 1S and 3P atomic orbital mix to form 4SP3 hybrid orbital .
Bond angle : Its bond angle is 109.5.
Shape : Its shape is Tetrahedral .
Example: Its example is Ethane (CH4) and Silicon tetra chloride.
How we can calculate hybridization?
This is very important question and answer to this question is .we can calculate hybridization by using the following formula
Hybridization = No of lone pairs +No of sigma bonds
For example in methane(CH4) carbon have 4 sigma bonds and no lone pair
So 4+ 0= 4
Its mean it has sp3 hybriidization
4=sp3
3=Sp2
2=Sp
Other methods are also used.



No comments:
Post a Comment